Photoelectron Spectroscopy Explain the Difference Between Energy Levels
67 By calculating and comparing the formation energy E form of various doping configurations one can theoretically deduce the preferential doping site. X-ray photoelectron spectroscopy XPS measurements were performed on a Thermo Scientific ESCALAB 250 spectrometer.

Photoelectron Spectra Following Excitation Of The 2 P O 4p 3 D 1 Download Scientific Diagram
7 and Table 2.
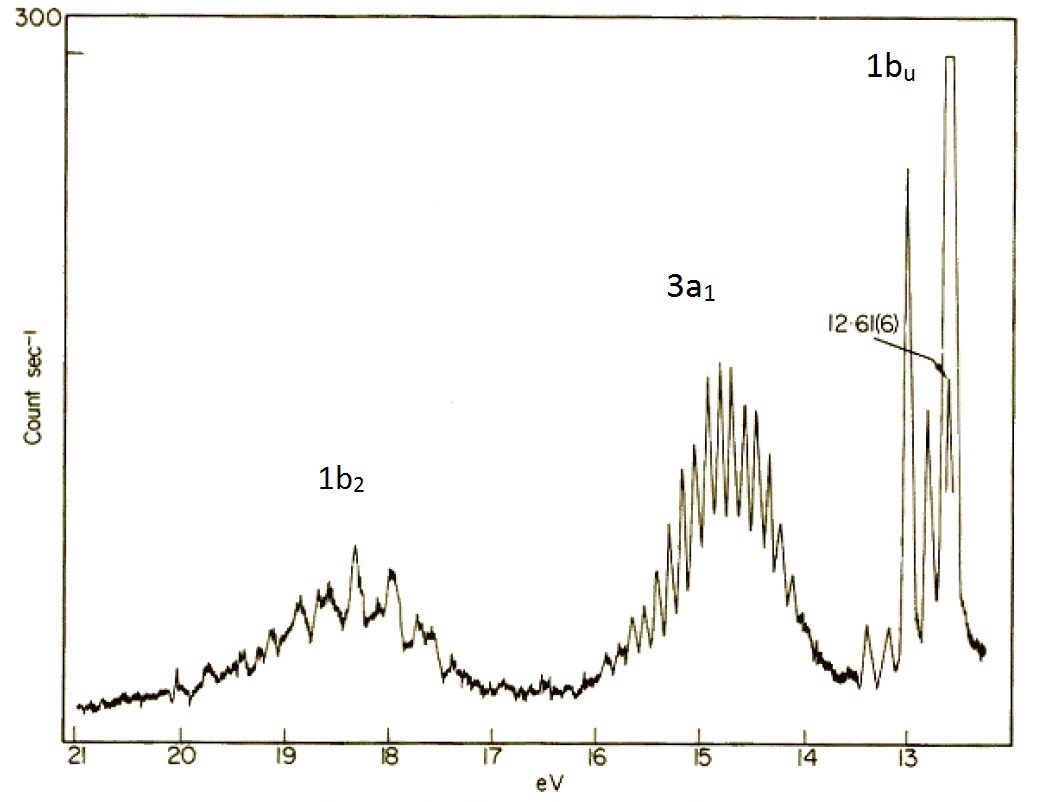
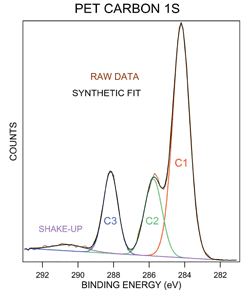
. Nevertheless the results obtained in. The experimental method to determine the real doping site is performing X-ray photoelectron spectroscopy to investigate the bonding state of the impurity element. Peak binding energies were referenced to the C 1s hydrocarbon peak at 2848 eV.
Because this energy is. The satellite emission lines do not correspond to the energy difference of two energy levels of the same atom instead they are transitions involving metalligand mixed molecular orbitals see Fig. Auger electron spectroscopy AES.
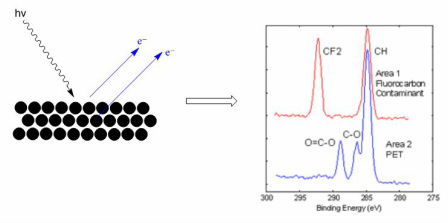
Pronounced in French is a common analytical technique used specifically in the study of surfaces and more generally in the area of materials scienceIt is a form of electron spectroscopy that relies on the Auger effect based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. Elemental analysis of surfaces in SEM is performed using energy dispersive spectroscopy EDS which measures the energy and intensity distribution of X-ray signals generated by the electron beam striking the surface of the specimen. Samples of the CeIV-rich and CeIII oleate precursors were prepared by dispersing the purified solid products in hexanes drop-casting on a conductive.
In solids the de Broglie wave of the photoelectron is then coherently scattered by the. A further analysis indicated that marine-current-energy implementation reduces the size of the daily energy-storage system by 79 in comparison to the use of only a photovoltaic system due to the similarity between the energy-demand profile and the marine-current-energy production profile. The results indicate that a greater participation of marine currents can help.
The measurement of these satellite emissions is sometimes also. The elemental composition at a point along a line or in a defined area can be. The inset therein is consistent with the 15 eV difference in binding energy spinorbit coupling between the 2p 12 and 2p 32 peaks of pure cobalt metal 4445.
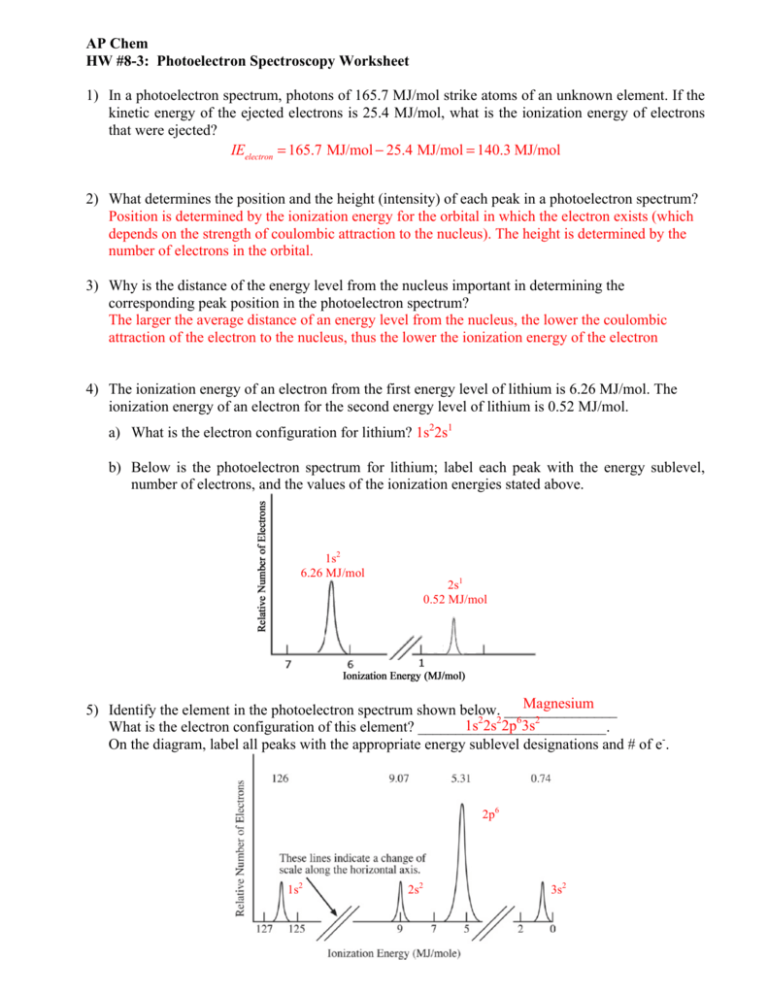
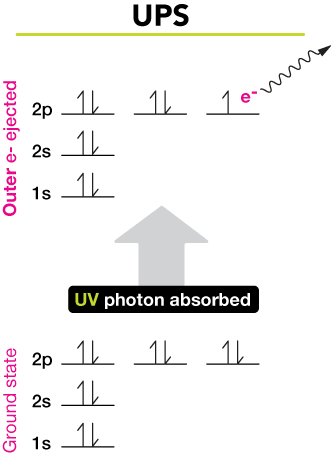
In optical spectroscopy the energy absorbed to move an electron to a more energetic level andor the energy emitted as the electron moves to a less energetic energy level is in the form of a photon a particle of light. These levels have well defined energies and electrons moving between them must absorb or emit energy equal to the difference between them.

13 Principle Of Photoelectron Spectroscopy Shown As An Example Is The Download Scientific Diagram
Photoelectron Spectroscopy Article Khan Academy

Photoelectron Spectroscopy An Overview Sciencedirect Topics

Photoelectron Spectroscopy Description Applications Video Lesson Transcript Study Com
10 4 Photoelectron Spectroscopy Chemistry Libretexts
4 12 Photoelectron Spectroscopy Pes Ups Xps Esca Chemistry Libretexts

Photoelectron Spectroscopy Quiz Flashcards Quizlet

Photoelectron Spectroscopy Article Khan Academy
Xps X Ray Photoelectron Spectroscopy Inolytix

Photoelectron Spectroscopy Pes A Technique Used To Measure The Binding Energy Of Electrons In An Atom Or Molecule As In The Photoelectric Effect Ppt Download

The Photoelectron Spectroscopy Pes Youtube

Hw 8 3 Photoelectron Spectroscopy Wks Key

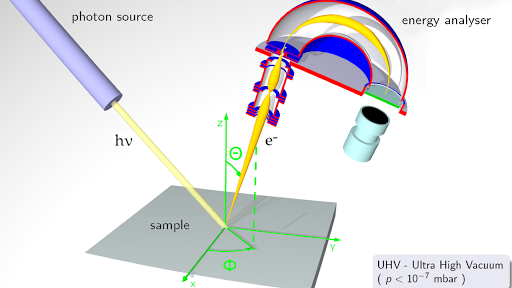
The Valence Band Photoelectron Spectrum Of Water Molecules Dot Ted Download Scientific Diagram
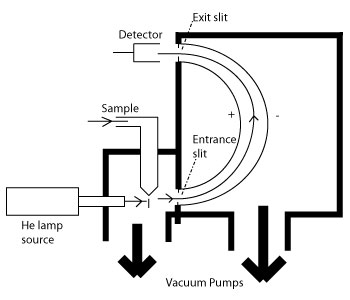
10 4 Photoelectron Spectroscopy Chemistry Libretexts
4 12 Photoelectron Spectroscopy Pes Ups Xps Esca Chemistry Libretexts
X Ray And Uv Photoelectron Spectroscopy Materials Science Nrel

Photoelectron Spectroscopy Overview Process Applications Video Lesson Transcript Study Com

Comments
Post a Comment